
Dr. Keith Reemtsma, a surgeon at Tulane University in New Orleans,
transplants thirteen chimpanzee kidneys into humans. Twelve of the patients
survive between nine and sixty days. One patient, however, survives for nine
months on primitive immunosuppression drugs with no signs of rejection. |
 |

While at the University of Colorado, Dr. Thomas Starzl transplants six
baboon kidneys into humans. Survival rates range between nineteen and 98 days,
with most patients dying of infections.
Dr. James Hardy of the University of Mississippi transplants a
chimpanzee heart into a 68-year old semi-comatose man. The chimpanzee heart is
too small to support the patient's circulatory system and only functions for
two hours.
|
 |

Dr. Christian Barnard, the South African surgeon who had performed the
first human heart allotransplant in 1967, attempts to use chimpanzee and baboon
hearts as bridge organs in patients who had undergone unsuccessful open heart
surgery. The recipient of the baboon heart dies after six hours, while the
recipient of the chimpanzee heart survives for four days before it is
rejected. |
 |
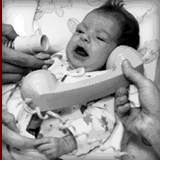

Dr. Leonard Bailey leads a group of surgeons who transplant a baboon
heart into a newborn infant, known as "Baby Fae," who was born with a poorly
developed left side of her heart. She is treated with cyclosporine, an
immunosuppressive drug that greatly increased survival rates in
allotransplants, and survives for twenty days before the heart is rejected.
Some have speculated that blood type incompatibility between Baby Fae and the
donor baboon may have played a role in the rejection process. |
 |

Dr. Thomas Starzl, now at the University of Pittsburgh, transplants a
baboon kidney into a patient with AIDS and hepatitis B, because it is believed
baboons are resistant to hepatitis B. The patient survives for 70 days, with
no evidence of rejection. He dies of an infection his body could not fight
off due to heavy immunosuppression. In the fall of 1999 it is discovered that
archived blood and tissue samples of the patient contained baboon
cytomegalovirus. It remains undetermined whether the virus had infected human
cells or whether baboon cells had migrated from the liver into other tissues.
A Polish surgeon transplants a pig heart into a human patient, who
survives for less than 24 hours. The cause of death is attributed to the small
size of the heart, which could not support the body's circulatory system.
On Christmas Eve, scientists at Imutran deliver the first transgenic
pig, which they name Astrid. The group of scientists, led by Dr. David White,
had inserted a small amount of human DNA into a fertilized pig egg, in an attempt to
create pig organs that would not be rejected by humans. |
 |

Dr. Thomas Starzl again attempts transplanting a baboon liver into a
patient suffering from hepatitis B. This patient never regains consciousness
after the operation, and dies of infection under heavy immunosuppression.
Starzl, who had received permission for several more xenotransplant operations,
halts his program to perform further research regarding transplant
rejection. |
 |

Scientists at Diacrin, Inc. receive FDA permission to begin clinical
trials using fetal pig neurons to treat patients suffering from Parkinson's
disease. These Phase 1 trials when concluded show efficacy and no safety
problems, leading to Phase 2 trials in the late 1990s.
In May, scientists from Nextran announce that they have developed
transgenic pig hearts that survive as long as 30 hours inside baboons, as
compared to the 60 to 90 minute survival time for regular pig hearts. In July,
the FDA approves Nextran's proposal to use transgenic pig livers as
bridge organs on up to ten patients.
AIDS patient Jeff Getty receives a transplant of baboon bone marrow cells
at San Francisco General Hospital, performed by Dr. Suzanne Ilstaad. Because
baboon stem cells are resistant to AIDS, the hope was that they would help
Getty's bone marrow produce AIDS-fighting immune cells. The baboon cells do
not take; they remain in Getty's system for only two weeks after the
transplant. He is still alive and blood tests so far have not revealed any
baboon viruses in his system. |
 |

Professor Robin Weiss discovers that viruses embedded in every pig cell
-- known as porcine endogenous retroviruses (PERV) -- can infect human cells in
culture. In the journal Nature he reports that each pig cell carries
approximately 50 copies of the PERV virus, and that up to three of them are
capable of infecting human cells. As a result, in October the FDA halts all
clinical trials until researchers can prove they have developed procedures to
detect low levels of PERV virus infection. The moratorium is lifted in January
1998.
|
 |

|
 |

The FDA effectively bans use of nonhuman primates in xenotransplants,
citing the risk of cross-species infection.
A study of 160 people who had received various pig tissues and/or cells
reveals that none had been infected with the PERV virus. The study was
conducted by researchers at Imutran, in collaboration with the CDC and reported
in the journal Science. |
 |

Scientists at PPL Therapeutics in Scotland announce in the journal
Nature that they have cloned five piglets for the first time. A team of
Japanese scientists announces in the journal Science that they have also
cloned a piglet using a different method.
Scientists at Infigin announce in the journal Nature
Biotechnology that they have produced two litters of transgenic, cloned
pigs.
In the journal Nature, Dr. Daniel Salomon of the Scripps
Research Institute announces the results of a study which found transmission of
the PERV virus during a transplant of pig pancreatic cells into heavily
immunosuppressed diabetic mice. This finding is the first evidence of
cross-species transmission of a retrovirus during a transplant. Salomon found
that the mice developed PERV infections that lasted for as long as two months
before going dormant.
The British animal rights organization Uncaged Campaigns receives
leaked documents of an Imutran study of the survivability of pig organs in
primates over a five-year period. The study showed the average survival time
was thirteen days, with a quarter of the primates dying within two days.
The International Society for Heart and Lung Transplantation issues a
report which advises that clinical xenotransplantation trials should not be
undertaken until authorities have determined a minimal virus risk, and until
60% of pig organs survive in non-human primates for a minimum of three months.
However, they conclude "Xenotransplantation has the potential to solve the
problem of donor organ supply, and therefore research in this field should be
actively encouraged and supported." |
 |

The FDA proposes a rule which would allow more public access to all new
or ongoing trials regarding xenotransplantation or gene therapy to ensure
public awareness of their unique potential for public health risks.
The United Kingdom Xenotransplantation Regulatory Authority (UKXIRA)
publishes its third annual report,
which states "Although alternative therapies are in development,
xenotransplantation may still offer the prospect of a viable treatment within a
worthwhile time frame. However, on the basis of current evidence, whole-organ
xenotransplantation, as a solution to the ongoing shortage of organs for
transplant, appears to be some way off." They conclude that they do not
support a moratorium on xenotransplantation, and that until the infection risk
is understood, they will assess particular procedures on a case-by-case
basis. |
 |

Preliminary analysis of Phase 2 controlled trials treating
Parkinson's disease patients with injected pig neuro cells indicate a setback.
Although there were improvements, the study found no difference in the improvements
between the patients who had been treated with the pig cells and those who had
a placebo treatment. |